光と色の話 第二部

第1回 光(電磁波)の吸収・透過・反射
「光と色の話(第一部)」の第1回~第3回でお話しましたように、「光」とは、物理的には電磁波の内の一種です。電磁波には波長が1 pm(=10-12 m)以下のガンマ線等から、波長が1 m を超える放送・通信用電波に至るまで、極めて広範囲の波長のものが知られていますが、その内で波長がおよそ10 nm~1 mm(1 nm=10-9 m)辺りの電磁波が“広義”の「光」とされ、またその中でも人間の眼に「明るさ」や「色」の感覚を引き起こす作用のある波長域(およそ380~780 nm)の領域が“狭義”の「光」(可視光または可視放射)です。
元々「光」とは、人間の眼に「明るさ」や「色」の感覚を引き起こすものとして認識され、これが本来の「光(可視光)」であったのですが、科学技術の発達によって、可視光の波長域の両側に眼には見えないけれども物理的には可視光と殆ど同じ特性の電磁波が存在することが解明され、「眼」という視点を離れて物理的視点からこれらを含めて「光」(広義の光)と言うようになった訳です。≪※1≫
人間生活に極めて大きな影響を及ぼす、可視光(狭義の光)の重要さは論を待ちませんが、紫外・赤外を含む広義の光もまた、直接的・間接的に様々な形で人間生活に影響を及ぼしています。これらの実際に観察される様々な現象・効果、例えば紫外放射による肌の日焼け、可視放射による物体の色、赤外放射による温熱効果、等々は、光の波長域に応じて色々と異なっており、それぞれの現象・効果の間には一見全く関連が無いようにも思えます。
しかし、これらの各種現象全体については、物質を構成する各種原子(原子核と電子)・分子群と光(電磁波)との相互作用の結果として統一的に論じることができます。
物体による光の反射・吸収・透過に関するエネルギー保存則
物体への入射光(放射束)は、微視的には物体を構成する物質(原子・分子)との相互作用の結果として、巨視的に捉えた「反射」、「吸収」、「透過」という三つの行先に分かれ、これらの間にはエネルギー保存則が成り立ちます。
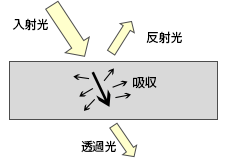
すなわち一般に
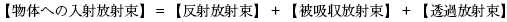
が成り立ちます。
これより、
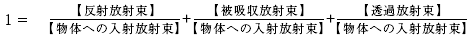
と書くことができますが、この三つの要素の相対比率が、物質の構成要素(原子・分子)、入射光の波長(振動数)、その他の条件(物体への入射角、物体の表面状態、等々)との相互関係に依存して変化することになります。(蛍光物質以外の)通常の物質ではこの関係が波長要素毎にも成立し、上式右辺の第1項が分光反射率R (λ)、第2項が分光吸収率A (λ)、第3項が分光透過率T (λ)として定義され、
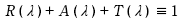
ということになります。
同一の白色光下の各種物体の色が異なって見えるのは、可視域における各物体の分光反射率R (λ) や分光透過率T(λ)が異なる結果として説明されます≪※ 第1部 第12回、13回≫。
つまり、物理現象である分光反射率R (λ)や分光透過率T (λ) を人間の眼で評価した結果の内の一つが「色」ということになりますが、この物理現象自体は可視域だけではなく当然のことながら赤外域、紫外域でも起こっています。そして、物質を構成する分子・原子と光との相互作用の内容は、光の波長(振動数)域によって様々で、その結果、表に現れる現象としては様々な異なる様相を呈しています。
物質による電磁波(光)の吸収
まず、光と物質との相互作用の一つに、物質による光の吸収が挙げられます。吸収という現象を例に、光(電磁波)と物質との相互作用について考えてみましょう。
光(電磁波)は波動性と粒子性を併せ持っており、粒子性に着目した光子(photon)のエネルギーE は振動数νに比例(波長λに反比例)しています。
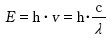
h:プランク定数( h = 6.626×10ー34 J・s )
c:真空中の光速 (c = 2.998×108 m/s )( c =ν・λ)
従って、光子エネルギーは、振動数が大きいほど(波長が短いほど)大きくなりますますので、光子エネルギーは 紫外 > 可視 > 赤外 という関係になっています。
一方、物質を構成する原子(原子核と電子)・分子は静止している訳ではなく、電子のエネルギー励起、分子を構成する原子間での振動、分子の回転、等々によって様々なエネルギー状態が存在します。
電磁波が物質に入射した時、物質の分子・原子のエネルギー状態が入射電磁波のエネルギー(振動する電場・磁場のエネルギー)と特定の関係にある場合には、共鳴相互作用が発生して電磁波の持つエネルギーが物質側へ受け渡されて物質側は活性化され、入射電磁波は消滅することになります。これが物質による電磁波の吸収です。
原子・分子のエネルギー状態としては大きく分類して以下の3種①②③があります。
①電子励起状態
物質を構成する原子内の電子のエネルギーは連続的な値をとることはなく、複数の離散的なエネルギー状態をとることが知られています。これを電子エネルギー準位と言い、最もエネルギーの小さい状態である基底準位(電子基底状態)とエネルギー的に活性化された状態である励起準位(電子励起状態)があります。
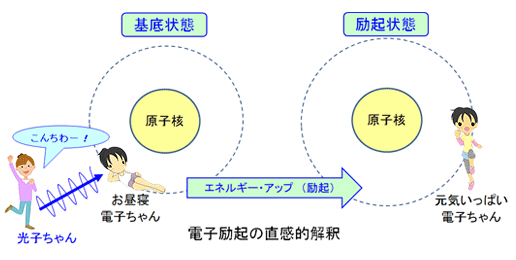
②振動励起状態
また更に、分子は複数個の原子が結合した構造であり、分子結合条件の下で振動をしており、この振動エネルギーについても振動基底状態と振動励起状態があります。
③回転励起状態
分子はまた分子構造を保ちながら全体として回転しています。この回転エネルギーについても回転基底状態と回転励起状態があります。
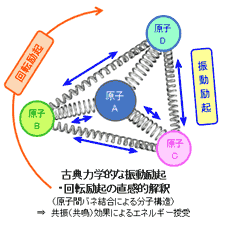
物質の種類毎に、それを構成する原子・分子の種類および組み合わせ(分子結合条件による分子振動や分子回転状態)は様々で、それに応じて①②③の各励起状態がとりうるエネルギー値はその物質固有の値をとることになります。その結果、その物質の原子・分子のその時のエネルギー状態に対して、入射電磁波の振動数(波長)が特定の関係にあれば、共鳴・相互作用によって電磁波から物質(原子・分子)へエネルギーの受け渡しが行われ、その結果電磁波は消滅することになります(物質による電磁波の吸収)。
これら3種のエネルギー状態は、①の電子励起状態が最も大きく、③の回転励起状態が最も小さくなっています。つまり、波長で考えると、
①の電子励起状態は、紫外~可視域の電磁波(光)の波長域のエネルギーに相当し、
②の振動励起状態は、中赤外~遠赤外の電磁波(光)の波長域のエネルギーに相当し、また
③の回転励起状態はもっと波長の長い「光」の領域を超えたマイクロ波や電波の領域の波長域のエネルギーに相当します。
温度というものの正体は、物質の分子・原子の振動活性化の程度を表すものとも言えます。大雑把なイメージとしては、温度が高いほど原子・分子の振動が活発であると考えて良いでしょう。理論的最低温度の絶対零度(0 K )では、原子振動が究極の最低状態ですが、この状態からエネルギーを得て原子振動が活発になるほど温度が上昇すると考えられています。この温度上昇という現象に最も関係の深いのが②の振動励起や③の回転励起です。例えば赤外線ホームコタツなどは、中赤外~遠赤外域の赤外線(赤外放射)のエネルギーを物体(身体や布団など)の原子・分子の振動エネルギーに吸収・変換させることによって温度上昇させているものです。
この振動励起や回転励起のエネルギー状態は、時間が経過すると、その励起エネルギーを電磁波(光)の形で外部に放出して元の基底状態に戻ることになります。このエネルギー放出が物質の温度低下(冷却)です。
一方、赤外よりも波長の短い可視~紫外放射の場合はどうでしょう。この領域の光子のエネルギーは、原子・分子の振動励起のエネルギーよりももっと大きく、主に電子励起のエネルギーに相当するする領域になります。つまり、(可視~近紫外の)光子が物質に入射すれば、物質内の電子を励起した結果として吸収という現象が表に出てきます。物質の種類毎にその構成原子・分子は様々ですので、電子励起に必要なエネルギーも様々な値になります。従って、電子励起に関与する光子の波長も物質の種類に対応して変わります。これが、物質の種類によって分光吸収率(その結果としての分光反射率、分光透過率)の違いとなって観察されることになります。
更に波長の短い(振動数の大きい)紫外域になってくると、光子エネルギーは電子励起エネルギーよりも大きくなってきて、電子励起では吸収しきれなくなってしまい、ついには物質の分子結合を破壊してしまうことにもなってきます。分子結合が破壊されるともう元には戻れず、物質自体の変質という結果になってしまいます。この効果は、可視域の短波長辺りから徐々に目立ち始め、近紫外から遠紫外へと、波長が短くなるほど顕著になってきます。我々人間は、真夏の直射日光の下で強い紫外線を浴びると皮膚が炎症を起こしたり、眼を痛めたりすることがありますが、これは日光に含まれる近紫外線(UV-A~UV-B)によって生体を形成する分子構造がダメージを受けることが原因になっています
≪※:第1部 第2回≫。また、紫外線殺菌灯はUV-Bよりももっと波長の短い紫外線UV-Cを使用して、バクテリアの核酸(DNA)を破壊して殺菌するものです≪※:第1部 第3回≫。これは当然、人体の皮膚や眼に対しても有害ですので、取り扱いに細心の注意が必要です。
電磁波吸収波長が物質の種類毎に異なることを利用して、分光吸収スペクトルの測定によって物質を分析・同定することも行われています。
電磁波(光)の透過と反射
一般に、可視域では不透明な物体が近赤外域では透過率が高くなっていることが多いと言われています。マシンビジョン分野ではこの現象を利用して検査・分析が行われることもよくあります。近赤外で透過率が高くなる、という現象は何故生じるのでしょうか?
電磁波(光)が物質を透過(および拡散反射)する現象に最も大きく関与しているのは 「レイリー散乱
( Rayleigh scattering )」 であると考えられます。レイリー散乱は、入射放射束の波長に対して粒子径が十分小さい場合に、波長の4乗に反比例(振動数の4乗に比例)して散乱を受ける(進行方向が変化する)、というものです。レイリー散乱は、気体や気体中に混在する微粒子による散乱を説明する場合(空が青い理由、夕日が赤い理由、etc.≪※ 第1部 第21回≫ )によく引用されますが、固体に対しても適用できます≪※2≫。
可視~近赤外の入射光が物質(原子・分子)の電子励起条件に合わない場合には吸収は起こらず、原子間をすり抜けたり、あるいは散乱されることになります。この場合、可視域よりも波長の長い赤外域の方が散乱を受けにくい、すなわち入射放射束の進行方向が変化を受けにくいため、 透過力が大きくなる、という定性的説明ができます≪※3≫。
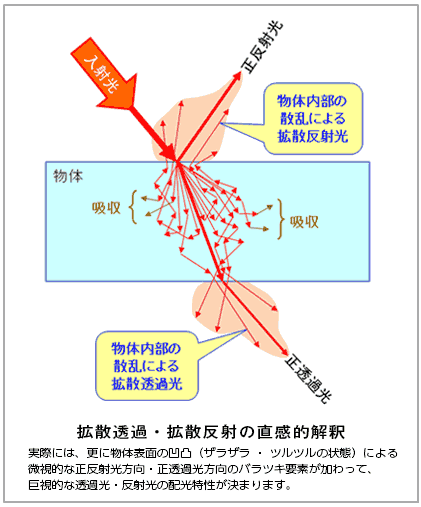
固体の場合には、物体表面の固体粒子(原子・分子)によって吸収されずに物体内部へ進行した光子(直進あるいは散乱された光子)も、物質を構成する粒子に次々と遭遇するため、波長が短い光子ほど指数関数的に多重に散乱を受けて急激に「直進成分」は減衰して進行方向が全方位均一化されていきます。波長が長い光子は散乱を受けにくいため、進行方向は短波長光子ほどには均一化されず、かなり「直進成分」が残ることになります。従って、(物体の厚みが薄い場合)直進成分の残っている長波長光子ほど物体の向こう側へ突き抜ける(透過する)光子が多くなると考えられます(正透過光)。散乱を受けて進行方向が変化した光子の内で、物体の向こう側へ放出される光子は、拡散透過光として観察されます。正透過光と拡散透過光で巨視的な透過光が形成されることになります。
物体表面で入射側空間の正反射方向に散乱された光子群(正反射光)に加えて、物体内部での散乱光子群の一部(入射側空間に再放出された拡散反射成分)が重なって、巨視的な反射光を構成することになります。
注釈
≪※1≫
日本語では、可視域の最短波長の紫よりも短い“紫の外側”という意味で紫外放射(紫外線)、可視域の最長波長の赤よりも長いという意味で赤外放射(赤外線)と呼んでいますが、これらに対応する英語は、紫外放射(紫外線)がUV(ultra-violet radiation)、赤外放射(赤外線)がIR(infra-red radiation)です。
ここで、英語の ”ultra-” および “infra-” という接頭語の意味を考えるとちょっと違和感を覚えます。
”ultra-” というのは、“~以上”とか、“~を超えて”という意味で、“infra-” というのは、“~以内”とか、“~未満”という意味の接頭語ですので、波長的には長短が逆の関係になっています。これは何故なのでしょうか?
現代において、我々は「光」の様々な特性を「波長」で議論することが多いため、このような疑問が生ずるのですが、可視域の外側に存在する広義の光(紫外放射や赤外放射)の存在が発見され、研究された歴史的経緯を考えれば、英語表現におけるこの接頭語の意味付けがわかってきます。
紫外放射や赤外放射が存在することの発見は、太陽光などの照射が身辺の各種物質に変化をもたらす現象の原因探究の結果として、これらの眼に見えない放射の存在に行き着いたということだと考えられます。つまり、光の物体への作用・効果は、その物体に照射される光の持つ「エネルギー」すなわち光の振動数によってその物体に対する影響効果の度合いが変わるということが研究の原点になっており、紫外放射は可視放射よりも光子エネルギーが大きく、紫の外側であるので ”ultra-violet“ 、赤外放射は可視放射よりも光子エネルギーが小さく、赤の外側であるので ”infra-red“ という名称が採用されたと考えられます。(ニュートン以来、18世紀頃までは、光は粒子として取り扱われることが多かったようです。)
≪※2≫
ただ、固体に対しては、散乱の原因となる粒子の密度が気体とは格段に異なること、また、金属か非金属か(自由電子を持つかどうか)によっても散乱に影響を及ぼす物理的挙動が異なり、波長の4乗に反比例という単純な論理だけで説明しきれるかどうかには議論の余地があるように思いますが、概ねそのような現象が起こっていると考えて良いと思われます。
≪※3≫
中赤外・遠赤外の領域になると、振動励起・回転励起の帯域になってくるため吸収が増加し、透過性が低下してくると考えられます。
光(電磁波)の吸収・透過・反射
Stories About Light and Color Part 2

Article 1 Light (Electromagnetic Wave) Absorption/ Transmission/ Reflection
As previously covered in articles 1 through 3 of “Stories About Light and Color (Part 1),” from a physical sciences standpoint, “light” is a type of electromagnetic wave. Although we are familiar with various of types of electromagnetic waves that possess an extremely broad range of wavelengths, ranging from gamma rays, etc., which possess a wavelength of 1pm (= 10-12 m) or shorter, to broadcasting/communication waves, which possess wavelengths that exceed 1m, “light” in the “broadest sense” of the term refers to electromagnetic waves that possess a wavelength roughly in the vicinity of 10 nm to 1 mm (1 nm = 10-9 m), while “light” in the “strictest sense” of the term (visible light or visible radiation), resides within the wavelength band which causes the human eye to sense “brightness” and “color” (roughly 380 to 780nm).
Originally, “light” was recognized as something that causes the human eye to sense such things as “brightness” and “color,” and while this equates to the traditional understanding of “light (visible light),” due to the advance of technology, it was discovered that there exist electromagnetic waves on either ends of the visible light spectrum, which are invisible to the naked eye, yet possess practically the same physical properties as visible light, thereby leading to the current understanding where less emphasis is placed on the “eye” itself, and where from a physical perspective, such waves have come to be recognized as a form of “light” (light in a broad sense). ≪*1≫
Although it need not be stressed how important visible light (light in a strict sense) is, due to its immense influence on our daily lives, light waves in the broad sense, which include ultraviolet/infrared light, also affect our daily lives in various ways in a direct/indirect manner. The various actually observable phenomena/effects of such waves, which can be seen in such things as the sunburn caused to our skin by ultraviolet radiation, the coloration of objects caused by visible radiation, and the hyperthermic effect of infrared rays, to name a few examples, all exhibit effects that vary widely depending on the wavelength band of the light in question, and at first glance, it may seem that such phenomena/effects are wholly unrelated with each other.
However, it is possible to provide a unified explanation for all such various phenomena by understanding them as the results of interactions between the various atomic particles (nuclei and electrons)/molecular groups that compose matter and light (electromagnetic waves).
The Law of Conservation of Energy as it Relates to Light Reflection/ Absorption/ Transmission by Objects
The incident light (radiant flux) of an object, from a micro standpoint can be understood as the result of interactions with the matter (atoms/molecules) that composes an object, and from a macro standpoint, such interactions can be divided into 3 types, namely, “reflection,” “absorption,” and “transmission,” and between these, the law of conservation of energy is upheld.

That is to say, generally speaking, the following holds true:
Object’s Incident Flux = Reflected Flux + Absorbed Flux + Transmitted Flux
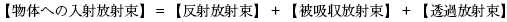
Based on this, it is possible to represent this relationship as follows:
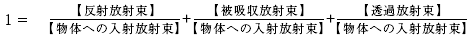
However, it must be noted that the relative proportion between these 3 factors will vary depending on the interrelationship between the composition of matter (atoms/molecules), incident light wavelength (oscillation frequency), and other conditions (angle of incidence for the object, the object’s surface conditions, etc.). For typical matter (excluding fluorescent matter), this relationship holds true for each wavelength constituent, which allows us to define the 1st member of the right side of the above equation as spectral reflectance R ( λ ), the 2nd member as the spectral absorption factor A ( λ ), and the 3rd member as spectral transmittance T ( λ ), thereby giving us the following equation:
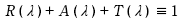
The variation in appearance of various objects under identical white lighting conditions can be explained as a result of differences within the visible range of spectral reflectance R ( λ ) and spectral transmittance T( λ ) for various objects ≪*Part 1 -Article 12 and Article 13 ≫. In other words, spectral reflectance R ( λ ) and spectral transmittance T ( λ ), which can be understood as physical phenomenon, cause the human eye to register a certain “color,” among other things, and as this physical phenomenon is not only limited to the visible range of light, it also occurs when processing light within the infrared band and ultraviolet band. Furthermore, the types of interactions between the molecules/atoms that compose matter and light vary widely depending on the wavelength range of the light (oscillation frequency), and as a result, the phenomena that manifest themselves through such interactions indeed exhibit a wide variety of appearances.
Absorption of Electromagnetic Waves (Light) by Matter
First, it is important to note that the absorption of light by matter is one of the types of interaction between light and matter. Using the example of the phenomenon of absorption, we will examine how light (electromagnetic waves) and matter interact with each other.
Light (electromagnetic waves) possesses both fluctuation and particulate characteristics, and by focusing on the particulate characteristics of photons, it can be observed that energy E is proportional to the oscillation frequency ν (inversely proportional to wavelength λ ).
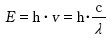
H: Planck’s constant ( h = 6.626×10-34 J・s )
C: Speed of light in a vacuum (c = 2.998×108 m/s) ( c = ν・λ )
For this reason, the larger the oscillation frequency (the shorter the wavelength), the larger the photon energy will become, and the relationship between photon energy can be understood as follows: ultraviolet > visible light > infrared.
With that said, it must be noted that the atoms (nuclei and electrons)/molecules that compose matter are not at rest, and that a variety of different types of energy states exist, such as that caused by the energy excitation of electrons, vibrations occurring between the atoms that compose the molecules, molecular rotation, etc.
When matter is subjected to incident electromagnetic waves, and for cases where there is a specific relationship between the molecular/atomic energy state of matter and the energy of the incident electromagnetic waves (energy of the vibrating electrical field/magnetic field), a resonance interaction will occur, causing the energy possessed by the electromagnetic wave to be transferred to the matter, thereby causing the matter to activate, resulting in elimination of the incident electromagnetic wave. This is referred to as the absorption of electromagnetic waves by matter.
The atomic/molecular energy state can be roughly divided into 3 types (①, ②, and ③), as shown below.
①Electron Excitation State
Electrons, contained within atoms that compose matter, possess energy that cannot be represented using a continuous value, and they are known to possess multiple, discrete energy states. This is referred to as the electron energy level, and it can be represented by the ground level, for which energy is the lowest (electron ground state), and the excited level, for which it is in an energetically activated state (electron excited state).

②Vibrational Excitation State
Furthermore, molecules are composed of a structure of multiple bonded atoms, which vibrate under molecular binding conditions, and this vibrational energy also possesses a vibrational ground state and vibrational excited state.
③Rotational Excitation State
Molecules also maintain their molecular structure as their entire structure rotates. This rotational energy also possesses a rotational ground state and a rotational excited state.

For each type of matter, the types of atoms/molecules they are composed of and the way in which they are combined (molecular vibrations and molecular rotation states caused by their molecular binding conditions) differ greatly, and accordingly, the energy value for the individual excitation states for ①, ②, and ③ will assume a value that is inherent to the type of matter in question. As a result, if a specified relationship exists between the current atomic/molecular energy state of the matter in question and the oscillation frequency (wavelength) of the incident electromagnetic wave, transference of energy from the electromagnetic wave to matter (atoms/molecules) caused by resonance/interactions will occur, resulting in the elimination of the electromagnetic wave (absorption of electromagnetic waves by matter).
Among these 3 types of energy states, the electron excitation state described in ① is the largest, and the rotational excitation state described in ③ is the smallest. In other words, from the perspective of wavelength, the following can be said:
The electron excitation state described in ① is equivalent to ultraviolet to visible light electromagnetic wave (light) wavelength band energy,
While the vibrational excitation state described in ② is equivalent to mid-infrared to far-infrared electromagnetic wave (light) wavelength band energy,
While the rotational excitation state described in ③ is equivalent to the wavelength band energy of microwaves and broadcasting waves that possess wavelengths that exceed the range of longer wavelength “light.”
Temperature, in its truest essence, can be understood as a representation of the degree of vibrational activation of the molecules/atoms of matter. In rough terms, it can be assumed that the higher the temperature is, the more active the atomic/molecular vibrations are. At the theoretically lowest temperature of absolute zero (0K), atomic vibrations are at their extreme lowest state, and from this state, it is understood that as energy is obtained and atomic vibrations become more active, temperature also increases. The phenomena that are most highly related to such temperature increases include vibrational excitation (②) and rotational excitation (③). For example, such things as home-use infrared heaters cause mid-infrared to far-infrared band infrared wave (infrared radiation) energy to be absorbed/converted into vibrational energy in objects (the human body or blankets, etc.), thereby resulting in increased temperatures.
Over time, such vibrational excitation and rotational excitation energy states will return to their original ground state, due to the external release of such excitation energy in the form of electromagnetic waves (light). This energy release results in temperature decreases in such matter (cooling).
On the other hand, what can be said about the visible light to ultraviolet radiation band, for which wavelengths are shorter than that of infrared light? The energy for photons within such ranges is much larger than the atomic/molecular vibrational excitation energy, and for this range, such energy is generally equivalent to electron excitation energy. In other words, if matter is subjected to incident photons (for the visible light to near-infrared range), the phenomenon of absorption occurs as a result of excitation of the electrons contained within the matter. Since the atoms/molecules that compose each individual type of matter vary greatly, the energy value necessary for electron excitation will also vary widely. For this reason, the wavelengths of the photons involved in electron excitation will vary depending on the type of matter present. This can be observed in the differences in the spectral absorption factor (resulting in differences in spectral reflectivity and spectral transmittance) seen for different types of matter.
For light within the ultraviolet range, for which wavelengths are even shorter (higher oscillation frequency), photon energy will become larger than electron excitation energy, and electron excitation will not be sufficient to absorb such energy, ultimately leading to destruction of the molecular bonds of the matter. If such molecular bonds are destroyed, they cannot be restored, and this will result in alteration of the matter. This effect gradually becomes noticeable in the short wavelength range of the visible light band, and as one moves from the near-ultraviolet to far-ultraviolet band, toward shorter wavelength ranges, this effect becomes more evident. For us humans, it is common for us to experience such things as skin inflammation or to sustain damage to the eyes when exposed to the strong ultraviolet rays present in the direct sunlight during mid-summer, and this damage to the molecular structures that form our body are caused by the near-ultraviolet rays (UV-A to UV-B) contained in sunlight ≪*: Part 1 - Article 2≫. Additionally, ultraviolet germicidal lamps make use of UV-C ultraviolet rays, which possess a wavelength that is much shorter than that of UV-B, in order to destroy the nucleic acids (DNA) contained in bacteria, thereby providing a sterilizing effect ≪*: Part 1 - Article 3≫. Such effects are, of course, harmful to the skin and eyes of the human body, and it is necessary to exercise great caution when handling such equipment.
By taking advantage of the fact that individual types of matter possess unique electromagnetic wave absorption wavelengths, it is also possible to perform analysis/identification of matter by means of spectroscopic absorption measurement.
Transmission and Reflection of Electromagnetic Waves (Light)
Generally speaking, for objects that are opaque within the range of visible light, it is said that transmittance often becomes higher at the near-infrared range. In the field of machine-vision technology, this phenomenon is frequently utilized in order to perform inspections/analysis. We must then ask the question, “why does this phenomenon occur whereby transmittance is higher for near-infrared ranges?”
“Rayleigh scattering” is considered to most significantly influence the phenomenon of electromagnetic wave (light) transmittance in matter (and diffuse reflectance). Rayleigh scattering refers to scattering (changes to the direction of travel) of light, in a manner that is inversely proportional to the fourth power of the wavelength (proportional to the fourth power of the oscillation frequency), for cases where particle size is sufficiently small in relation to the wavelength of the incident flux. Although Rayleigh scattering is often cited to explain the diffusion of minute particles that intermingle with gases and within gaseous bodies (the reason why the sky is blue, reason why sunsets are red, etc., ≪*Series 1 - Article 21≫), the principle can also be applied to solids as well ≪*2≫.
For cases where visible light to near-infrared range incident light does not satisfy the electron excitation conditions of matter (atoms/molecules), absorption will not occur, causing light to pass between individual atoms, or resulting in scattering. In such cases, infrared range light, which possesses a longer wavelength than that of the visible light range, is less susceptible to scattering, providing us with the qualitative explanation that transmittance becomes higher due to the fact that the direction of travel of the incident flux is less susceptible to alteration ≪*3≫.

拡散透過・拡散反射の直感的解釈
In practice, transmitted light/reflected light from a macroscopic standpoint is determined based on the microscopic variations in regular reflected light direction/regular transmitted light direction factors caused by indentations on the surface of the object (whether the surface is grainy/smooth).
In the case of solids, photons that have not been absorbed by solid particles (atoms/molecules) on an object’s surface, and that have internally penetrated an object (photons that pass straight through or are scattered) also encounter the particles that compose matter, in rapid succession, and for such photons, the shorter the wavelength, the more such photons are subjected to exponential multiple scattering, leading to drastic attenuation of “directly-transmitted components,” which in turn results in increased omni-directional uniformity of the direction of travel. Since photons with a long wavelength are less susceptible to scattering, the direction of travel does not become as uniform as for that of short wavelength photons, and this results in the presence of a considerable amount of residual “directly-transmitted components.” By this reasoning, for longer wavelength photons, for which more directly-transmitted components remain (for cases where the object is thin), the amount of photons that penetrate (transmit) through to the other side of the object are higher (regularly transmitted light). Among those photons, which undergo changes to their direction of travel due to the effects of scattering, those that are emitted through to the other side of the object can be observed as diffused transmitted light. In such a way, transmitted light, in a macro sense, is composed of regularly transmitted light and diffused transmitted light.
In addition to the bundle of photons (regularly transmitted light) that are scattered toward the regular reflection direction of the incident-side space on the object surface, a portion of the scattered photon bundle (diffused reflected components re-emitted within the incident-side space) will overlap within the object, forming reflected light, in the macroscopic sense of the word.
Notes
≪*1≫
In Japanese, the term ultraviolet radiation (ultraviolet rays) is used to indicate light that is “outside of the violet range” (violet being the shortest wavelength light within the visible range of light), and infrared radiation (infrared rays) is used to indicate light that possesses a wavelength that is longer than red light (which is the longest wavelength light within the visible range of light), whereas in the English language, the terms UV (ultraviolet radiation) and IR (infrared radiation) are used.
When examining these terms, there is a slight sense of incongruity with the usage of the English prefixes “ultra-”and “infra-.” Although the prefix “ultra-” can mean “equal to or more” or “beyond,” while “infra-” can mean “at or below” or “less than,” the usage here of these prefixes in relation to wavelength is reversed. Why is this?
In modern times, mankind has frequently engaged in discussions on the various characteristics of light, in terms of “wavelength,” causing such questions to arise, however, by examining the historical background within which the existence of light (in the broadest sense of the term) outside of the visible range of light (ultraviolet radiation and infrared radiation) was discovered and researched, the meanings of these English prefixes become more apparent.
The discovery of the existence of ultraviolet radiation and infrared radiation is a result of explorations into the cause of phenomena whereby various types of everyday matter undergo changes when exposed to irradiation by sunlight, etc., and such explorations eventually led to the discovery of such forms of radiation that are invisible to the naked eye. In other words, research on the interactions/effects of light on objects traces its origins to studies on how the degree of this effect on an object varies in accordance with the “energy” of the light the object is irradiated with (oscillation frequency of light), hence, the term “ultra-violet” was chosen to indicate that ultraviolet radiation possesses photon energy larger than that of visible radiation, and that it is outside of the range of violet light, and it is thought that the term “infra-red” was chosen to indicate that infrared radiation possesses photon energy that is smaller than that of visible light, and that it is outside of the range of red light (since the days of Newton, until roughly the 18th century, light was often considered to be a type of particle).
≪*2≫
It must be noted, however, that for solids, the density of particles that result in scattering is significantly different from that found for gaseous bodies, and furthermore, depending on whether the matter is metallic or non-metallic (whether it possesses free electrons or not), such physical behavior affecting scattering will differ, leading to questions as to whether one can rely on the simple theory that scattering is inversely proportional to the fourth power of the wavelength, but for our purposes, it should be sufficient to assume that such phenomenon is occurring under such general conditions.
≪*3≫
For light that is within the range of the mid-infrared/far-infrared range, absorption is thought to increase since this corresponds with the vibrational excitation/rotational excitation range, leading to a decrease in transmittance.
Light (Electromagnetic Wave) Absorption/Transmission/Reflection
Stories About Light and Color Part 2

Article 1 Light (Electromagnetic Wave) Absorption/ Transmission/ Reflection
As previously covered in articles 1 through 3 of “Stories About Light and Color (Part 1),” from a physical sciences standpoint, “light” is a type of electromagnetic wave. Although we are familiar with various of types of electromagnetic waves that possess an extremely broad range of wavelengths, ranging from gamma rays, etc., which possess a wavelength of 1pm (= 10-12 m) or shorter, to broadcasting/communication waves, which possess wavelengths that exceed 1m, “light” in the “broadest sense” of the term refers to electromagnetic waves that possess a wavelength roughly in the vicinity of 10 nm to 1 mm (1 nm = 10-9 m), while “light” in the “strictest sense” of the term (visible light or visible radiation), resides within the wavelength band which causes the human eye to sense “brightness” and “color” (roughly 380 to 780nm).
Originally, “light” was recognized as something that causes the human eye to sense such things as “brightness” and “color,” and while this equates to the traditional understanding of “light (visible light),” due to the advance of technology, it was discovered that there exist electromagnetic waves on either ends of the visible light spectrum, which are invisible to the naked eye, yet possess practically the same physical properties as visible light, thereby leading to the current understanding where less emphasis is placed on the “eye” itself, and where from a physical perspective, such waves have come to be recognized as a form of “light” (light in a broad sense). ≪*1≫
Although it need not be stressed how important visible light (light in a strict sense) is, due to its immense influence on our daily lives, light waves in the broad sense, which include ultraviolet/infrared light, also affect our daily lives in various ways in a direct/indirect manner. The various actually observable phenomena/effects of such waves, which can be seen in such things as the sunburn caused to our skin by ultraviolet radiation, the coloration of objects caused by visible radiation, and the hyperthermic effect of infrared rays, to name a few examples, all exhibit effects that vary widely depending on the wavelength band of the light in question, and at first glance, it may seem that such phenomena/effects are wholly unrelated with each other.
However, it is possible to provide a unified explanation for all such various phenomena by understanding them as the results of interactions between the various atomic particles (nuclei and electrons)/molecular groups that compose matter and light (electromagnetic waves).
The Law of Conservation of Energy as it Relates to Light Reflection/ Absorption/ Transmission by Objects
The incident light (radiant flux) of an object, from a micro standpoint can be understood as the result of interactions with the matter (atoms/molecules) that composes an object, and from a macro standpoint, such interactions can be divided into 3 types, namely, “reflection,” “absorption,” and “transmission,” and between these, the law of conservation of energy is upheld.

That is to say, generally speaking, the following holds true:
Object’s Incident Flux = Reflected Flux + Absorbed Flux + Transmitted Flux
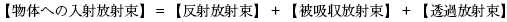
Based on this, it is possible to represent this relationship as follows:
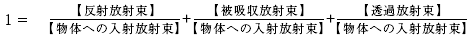
However, it must be noted that the relative proportion between these 3 factors will vary depending on the interrelationship between the composition of matter (atoms/molecules), incident light wavelength (oscillation frequency), and other conditions (angle of incidence for the object, the object’s surface conditions, etc.). For typical matter (excluding fluorescent matter), this relationship holds true for each wavelength constituent, which allows us to define the 1st member of the right side of the above equation as spectral reflectance R ( λ ), the 2nd member as the spectral absorption factor A ( λ ), and the 3rd member as spectral transmittance T ( λ ), thereby giving us the following equation:
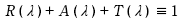
The variation in appearance of various objects under identical white lighting conditions can be explained as a result of differences within the visible range of spectral reflectance R ( λ ) and spectral transmittance T( λ ) for various objects ≪*Part 1 -Article 12 and Article 13 ≫. In other words, spectral reflectance R ( λ ) and spectral transmittance T ( λ ), which can be understood as physical phenomenon, cause the human eye to register a certain “color,” among other things, and as this physical phenomenon is not only limited to the visible range of light, it also occurs when processing light within the infrared band and ultraviolet band. Furthermore, the types of interactions between the molecules/atoms that compose matter and light vary widely depending on the wavelength range of the light (oscillation frequency), and as a result, the phenomena that manifest themselves through such interactions indeed exhibit a wide variety of appearances.
Absorption of Electromagnetic Waves (Light) by Matter
First, it is important to note that the absorption of light by matter is one of the types of interaction between light and matter. Using the example of the phenomenon of absorption, we will examine how light (electromagnetic waves) and matter interact with each other.
Light (electromagnetic waves) possesses both fluctuation and particulate characteristics, and by focusing on the particulate characteristics of photons, it can be observed that energy E is proportional to the oscillation frequency ν (inversely proportional to wavelength λ ).
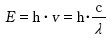
H: Planck’s constant ( h = 6.626×10-34 J・s )
C: Speed of light in a vacuum (c = 2.998×108 m/s) ( c = ν・λ )
For this reason, the larger the oscillation frequency (the shorter the wavelength), the larger the photon energy will become, and the relationship between photon energy can be understood as follows: ultraviolet > visible light > infrared.
With that said, it must be noted that the atoms (nuclei and electrons)/molecules that compose matter are not at rest, and that a variety of different types of energy states exist, such as that caused by the energy excitation of electrons, vibrations occurring between the atoms that compose the molecules, molecular rotation, etc.
When matter is subjected to incident electromagnetic waves, and for cases where there is a specific relationship between the molecular/atomic energy state of matter and the energy of the incident electromagnetic waves (energy of the vibrating electrical field/magnetic field), a resonance interaction will occur, causing the energy possessed by the electromagnetic wave to be transferred to the matter, thereby causing the matter to activate, resulting in elimination of the incident electromagnetic wave. This is referred to as the absorption of electromagnetic waves by matter.
The atomic/molecular energy state can be roughly divided into 3 types (①, ②, and ③), as shown below.
①Electron Excitation State
Electrons, contained within atoms that compose matter, possess energy that cannot be represented using a continuous value, and they are known to possess multiple, discrete energy states. This is referred to as the electron energy level, and it can be represented by the ground level, for which energy is the lowest (electron ground state), and the excited level, for which it is in an energetically activated state (electron excited state).

②Vibrational Excitation State
Furthermore, molecules are composed of a structure of multiple bonded atoms, which vibrate under molecular binding conditions, and this vibrational energy also possesses a vibrational ground state and vibrational excited state.
③Rotational Excitation State
Molecules also maintain their molecular structure as their entire structure rotates. This rotational energy also possesses a rotational ground state and a rotational excited state.

For each type of matter, the types of atoms/molecules they are composed of and the way in which they are combined (molecular vibrations and molecular rotation states caused by their molecular binding conditions) differ greatly, and accordingly, the energy value for the individual excitation states for ①, ②, and ③ will assume a value that is inherent to the type of matter in question. As a result, if a specified relationship exists between the current atomic/molecular energy state of the matter in question and the oscillation frequency (wavelength) of the incident electromagnetic wave, transference of energy from the electromagnetic wave to matter (atoms/molecules) caused by resonance/interactions will occur, resulting in the elimination of the electromagnetic wave (absorption of electromagnetic waves by matter).
Among these 3 types of energy states, the electron excitation state described in ① is the largest, and the rotational excitation state described in ③ is the smallest. In other words, from the perspective of wavelength, the following can be said:
The electron excitation state described in ① is equivalent to ultraviolet to visible light electromagnetic wave (light) wavelength band energy,
While the vibrational excitation state described in ② is equivalent to mid-infrared to far-infrared electromagnetic wave (light) wavelength band energy,
While the rotational excitation state described in ③ is equivalent to the wavelength band energy of microwaves and broadcasting waves that possess wavelengths that exceed the range of longer wavelength “light.”
Temperature, in its truest essence, can be understood as a representation of the degree of vibrational activation of the molecules/atoms of matter. In rough terms, it can be assumed that the higher the temperature is, the more active the atomic/molecular vibrations are. At the theoretically lowest temperature of absolute zero (0K), atomic vibrations are at their extreme lowest state, and from this state, it is understood that as energy is obtained and atomic vibrations become more active, temperature also increases. The phenomena that are most highly related to such temperature increases include vibrational excitation (②) and rotational excitation (③). For example, such things as home-use infrared heaters cause mid-infrared to far-infrared band infrared wave (infrared radiation) energy to be absorbed/converted into vibrational energy in objects (the human body or blankets, etc.), thereby resulting in increased temperatures.
Over time, such vibrational excitation and rotational excitation energy states will return to their original ground state, due to the external release of such excitation energy in the form of electromagnetic waves (light). This energy release results in temperature decreases in such matter (cooling).
On the other hand, what can be said about the visible light to ultraviolet radiation band, for which wavelengths are shorter than that of infrared light? The energy for photons within such ranges is much larger than the atomic/molecular vibrational excitation energy, and for this range, such energy is generally equivalent to electron excitation energy. In other words, if matter is subjected to incident photons (for the visible light to near-infrared range), the phenomenon of absorption occurs as a result of excitation of the electrons contained within the matter. Since the atoms/molecules that compose each individual type of matter vary greatly, the energy value necessary for electron excitation will also vary widely. For this reason, the wavelengths of the photons involved in electron excitation will vary depending on the type of matter present. This can be observed in the differences in the spectral absorption factor (resulting in differences in spectral reflectivity and spectral transmittance) seen for different types of matter.
For light within the ultraviolet range, for which wavelengths are even shorter (higher oscillation frequency), photon energy will become larger than electron excitation energy, and electron excitation will not be sufficient to absorb such energy, ultimately leading to destruction of the molecular bonds of the matter. If such molecular bonds are destroyed, they cannot be restored, and this will result in alteration of the matter. This effect gradually becomes noticeable in the short wavelength range of the visible light band, and as one moves from the near-ultraviolet to far-ultraviolet band, toward shorter wavelength ranges, this effect becomes more evident. For us humans, it is common for us to experience such things as skin inflammation or to sustain damage to the eyes when exposed to the strong ultraviolet rays present in the direct sunlight during mid-summer, and this damage to the molecular structures that form our body are caused by the near-ultraviolet rays (UV-A to UV-B) contained in sunlight ≪*: Part 1 - Article 2≫. Additionally, ultraviolet germicidal lamps make use of UV-C ultraviolet rays, which possess a wavelength that is much shorter than that of UV-B, in order to destroy the nucleic acids (DNA) contained in bacteria, thereby providing a sterilizing effect ≪*: Part 1 - Article 3≫. Such effects are, of course, harmful to the skin and eyes of the human body, and it is necessary to exercise great caution when handling such equipment.
By taking advantage of the fact that individual types of matter possess unique electromagnetic wave absorption wavelengths, it is also possible to perform analysis/identification of matter by means of spectroscopic absorption measurement.
Transmission and Reflection of Electromagnetic Waves (Light)
Generally speaking, for objects that are opaque within the range of visible light, it is said that transmittance often becomes higher at the near-infrared range. In the field of machine-vision technology, this phenomenon is frequently utilized in order to perform inspections/analysis. We must then ask the question, “why does this phenomenon occur whereby transmittance is higher for near-infrared ranges?”
“Rayleigh scattering” is considered to most significantly influence the phenomenon of electromagnetic wave (light) transmittance in matter (and diffuse reflectance). Rayleigh scattering refers to scattering (changes to the direction of travel) of light, in a manner that is inversely proportional to the fourth power of the wavelength (proportional to the fourth power of the oscillation frequency), for cases where particle size is sufficiently small in relation to the wavelength of the incident flux. Although Rayleigh scattering is often cited to explain the diffusion of minute particles that intermingle with gases and within gaseous bodies (the reason why the sky is blue, reason why sunsets are red, etc., ≪*Series 1 - Article 21≫), the principle can also be applied to solids as well ≪*2≫.
For cases where visible light to near-infrared range incident light does not satisfy the electron excitation conditions of matter (atoms/molecules), absorption will not occur, causing light to pass between individual atoms, or resulting in scattering. In such cases, infrared range light, which possesses a longer wavelength than that of the visible light range, is less susceptible to scattering, providing us with the qualitative explanation that transmittance becomes higher due to the fact that the direction of travel of the incident flux is less susceptible to alteration ≪*3≫.

拡散透過・拡散反射の直感的解釈
In practice, transmitted light/reflected light from a macroscopic standpoint is determined based on the microscopic variations in regular reflected light direction/regular transmitted light direction factors caused by indentations on the surface of the object (whether the surface is grainy/smooth).
In the case of solids, photons that have not been absorbed by solid particles (atoms/molecules) on an object’s surface, and that have internally penetrated an object (photons that pass straight through or are scattered) also encounter the particles that compose matter, in rapid succession, and for such photons, the shorter the wavelength, the more such photons are subjected to exponential multiple scattering, leading to drastic attenuation of “directly-transmitted components,” which in turn results in increased omni-directional uniformity of the direction of travel. Since photons with a long wavelength are less susceptible to scattering, the direction of travel does not become as uniform as for that of short wavelength photons, and this results in the presence of a considerable amount of residual “directly-transmitted components.” By this reasoning, for longer wavelength photons, for which more directly-transmitted components remain (for cases where the object is thin), the amount of photons that penetrate (transmit) through to the other side of the object are higher (regularly transmitted light). Among those photons, which undergo changes to their direction of travel due to the effects of scattering, those that are emitted through to the other side of the object can be observed as diffused transmitted light. In such a way, transmitted light, in a macro sense, is composed of regularly transmitted light and diffused transmitted light.
In addition to the bundle of photons (regularly transmitted light) that are scattered toward the regular reflection direction of the incident-side space on the object surface, a portion of the scattered photon bundle (diffused reflected components re-emitted within the incident-side space) will overlap within the object, forming reflected light, in the macroscopic sense of the word.
Notes
≪*1≫
In Japanese, the term ultraviolet radiation (ultraviolet rays) is used to indicate light that is “outside of the violet range” (violet being the shortest wavelength light within the visible range of light), and infrared radiation (infrared rays) is used to indicate light that possesses a wavelength that is longer than red light (which is the longest wavelength light within the visible range of light), whereas in the English language, the terms UV (ultraviolet radiation) and IR (infrared radiation) are used.
When examining these terms, there is a slight sense of incongruity with the usage of the English prefixes “ultra-”and “infra-.” Although the prefix “ultra-” can mean “equal to or more” or “beyond,” while “infra-” can mean “at or below” or “less than,” the usage here of these prefixes in relation to wavelength is reversed. Why is this?
In modern times, mankind has frequently engaged in discussions on the various characteristics of light, in terms of “wavelength,” causing such questions to arise, however, by examining the historical background within which the existence of light (in the broadest sense of the term) outside of the visible range of light (ultraviolet radiation and infrared radiation) was discovered and researched, the meanings of these English prefixes become more apparent.
The discovery of the existence of ultraviolet radiation and infrared radiation is a result of explorations into the cause of phenomena whereby various types of everyday matter undergo changes when exposed to irradiation by sunlight, etc., and such explorations eventually led to the discovery of such forms of radiation that are invisible to the naked eye. In other words, research on the interactions/effects of light on objects traces its origins to studies on how the degree of this effect on an object varies in accordance with the “energy” of the light the object is irradiated with (oscillation frequency of light), hence, the term “ultra-violet” was chosen to indicate that ultraviolet radiation possesses photon energy larger than that of visible radiation, and that it is outside of the range of violet light, and it is thought that the term “infra-red” was chosen to indicate that infrared radiation possesses photon energy that is smaller than that of visible light, and that it is outside of the range of red light (since the days of Newton, until roughly the 18th century, light was often considered to be a type of particle).
≪*2≫
It must be noted, however, that for solids, the density of particles that result in scattering is significantly different from that found for gaseous bodies, and furthermore, depending on whether the matter is metallic or non-metallic (whether it possesses free electrons or not), such physical behavior affecting scattering will differ, leading to questions as to whether one can rely on the simple theory that scattering is inversely proportional to the fourth power of the wavelength, but for our purposes, it should be sufficient to assume that such phenomenon is occurring under such general conditions.
≪*3≫
For light that is within the range of the mid-infrared/far-infrared range, absorption is thought to increase since this corresponds with the vibrational excitation/rotational excitation range, leading to a decrease in transmittance.
Light (Electromagnetic Wave) Absorption/Transmission/Reflection

